
![AdobeStock_541064000-[Converted]](http://heartcorsolutions.com/wp-content/plugins/revslider/sr6/assets/assets/dummy.png)
Blue Spark Technologies, Inc., and the PCA 500 from QT Medical.


 View our complete conference schedule
View our complete conference schedule
our next event



Who we work with
Pharmaceutical Companies
In early or pivotal study phases preparing IND filings and conducting post-market surveillance
Medical Device Companies
Large and early stage, preparing PMA submissions, as well as post-market surveillance studies
Contract Research Organizations (CROs)
Whose pharmaceutical or medical device sponsors need expert evidence of cardiac rhythm safety for their client company's regulatory filings and ongoing support studies
What sets HeartcoR apart
Cardiac Arrhythmia data collection occurs anywhere our customers need




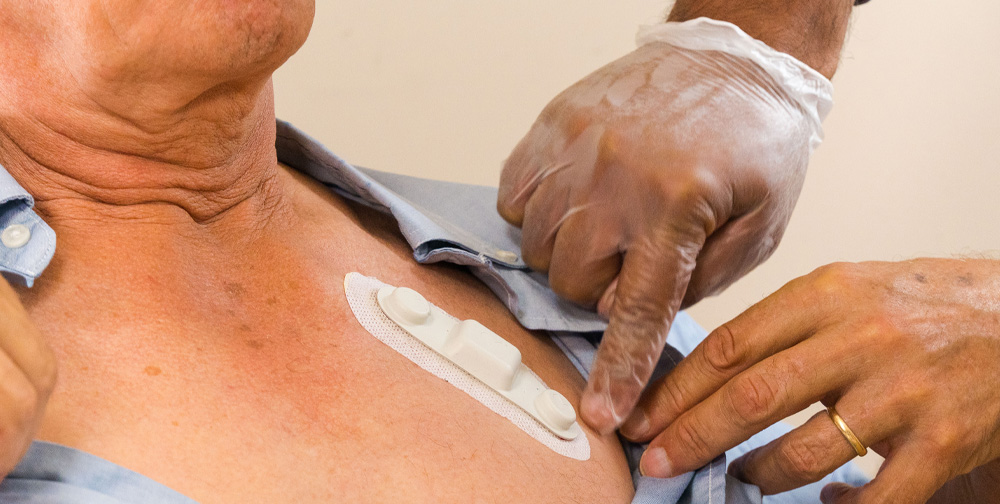

ECG modes we collect
Each HeartcoR customer’s clinical study protocol differs in design. To meet our customer’s requirements, we collect ECG data in four modes within study-specified time periods. ECG collection modes are defined by the time required for capture and reporting of a cardiac rhythm segment.
Usually 60-second recordings that monitor the effect of a treatment, post therapy. Our event collections are accomplished with an easy-to-use handheld device actuated by an application situated on a locked cell phone provided by HeartcoR.
Mobile Cardiac Telemetry (MCT) is a real-time ECG collection mode technology that may be prescribed in a clinical study.
Longer than event recordings, Holter recordings are typically 24 hour, continuous cardiac rhythm collections for assessing cardiac reactions to a therapy over a longer timeframe. HeartcoR Holter recordings are collected by a small, hydrogel patch electrode easily positioned on the patient’s chest. The patch electrode is actuated by a separate application on the locked cell phone provided by HeartcoR to study participants.
12-lead ECGs use 10 electrodes to produce 12 different views of the heart’s electrical activity. 12-lead ECGs are typically collected from a patient at rest as a snapshot of electrical heart activity. HeartcoR uses the latest 12-lead system for data collection when such studies are required by a clinical protocol.
Leading healthcare companies choose HeartcoR Solutions
HeartcoR is the top choice for four of the largest global healthcare companies, as well as startups seeking initial market approval because we deliver:
High-touch customer service
leading to high patient adherence.
On-time, on-budget
project delivery.
Cutting-edge data capture devices
and ECG analysis software technology solutions.
Protocol-specific and customized data
reporting for study progress tracking.
A deep bench of ECG-certified technicians,
QA oversight, and board-certified electrophysiologists to conduct tracing analysis.
“Thank you HeartcoR team for giving great service to our sites!”
“Amidst the busyness on both sides, it is very important to recognize what is working well as we continue to work on fixing the other ongoing issues. I wanted to take a moment to thank your team’s efforts in trying to get our company’s data review back on track. Special kudos to Destinee who seems to have offered excellent customer service to one of our participating sites.”

“Through the years I have been part of many studies and have been reintroduced to my HeartcoR contact who greeted me with the kindest, most welcome feeling. He’s never flustered or bothered by my many emails and requests. I am not sure what official position he holds within your company, but I know he exhibits the intelligence, kindness, and thoroughness of a true leader.”